
HTRF Human Total YES Detection Kit, 10,000 assay points
HTRF Human Total YES Detection Kit, 10,000 assay points


This HTRF kit allows for the cell-based quantitative detection of Total YES.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
This HTRF kit allows for the cell-based quantitative detection of Total YES.


HTRF Human Total YES Detection Kit, 10,000 assay points


HTRF Human Total YES Detection Kit, 10,000 assay points


Product information
Overview
YES,also known as YES1 which belongs to the Src family of kinases, is a proto-oncogene and a non-receptor tyrosine kinase located at the plasma membrane. Once activated, YES phosphorylates various downstream effectors including LATS1/2, YAP, FAK, Caveolin-1, and p130CAS, as well as Crk. The kinase contributes to a wide range of cellular processes, including cell proliferation, survival, cytoskeleton rearrangements, migration, and adhesion. Dysregulation of YES can contribute to pathological conditions, especially in the context of cancer.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 4
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
YES
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Inflammation
Oncology
|
| Unit Size |
10,000 assay points
|
How it works
Total YES assay principle
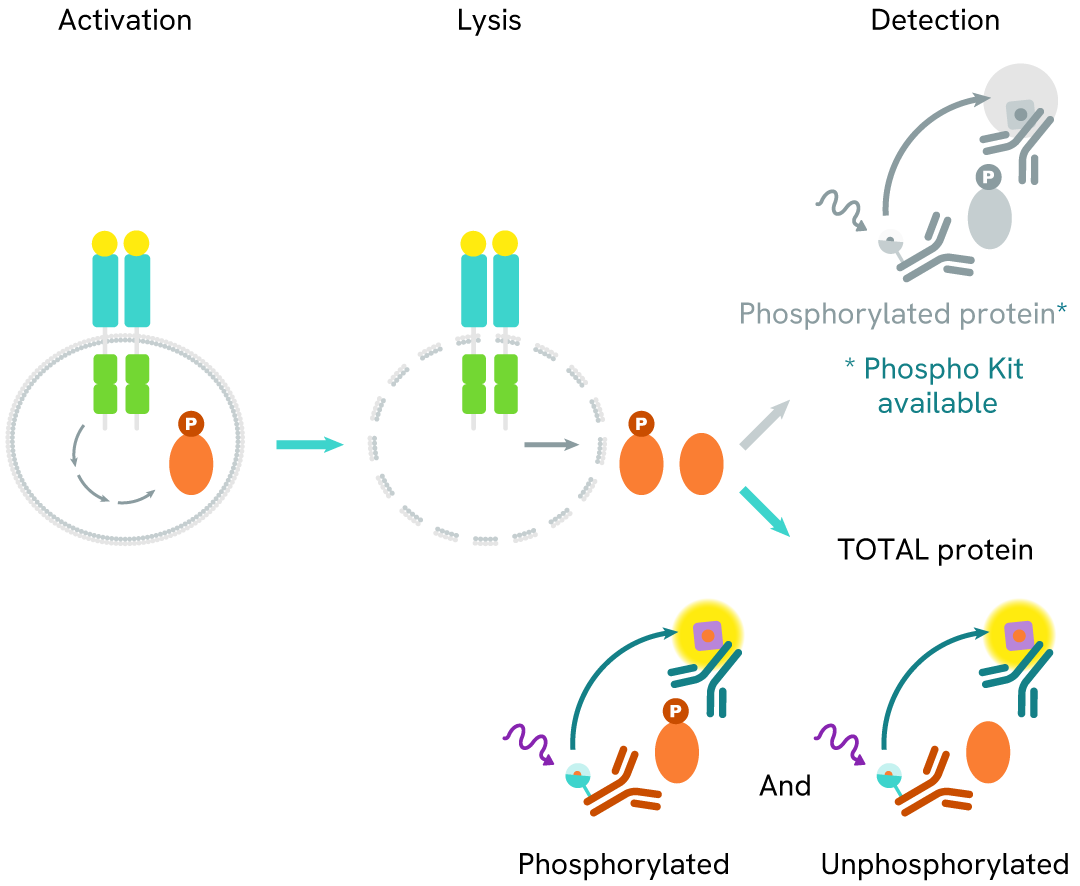
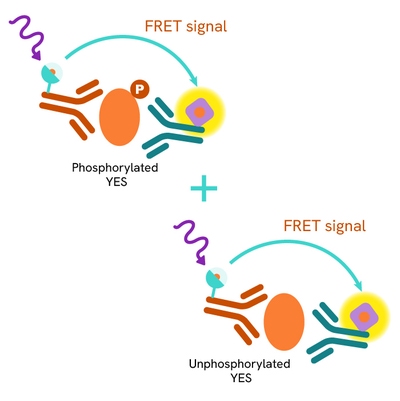
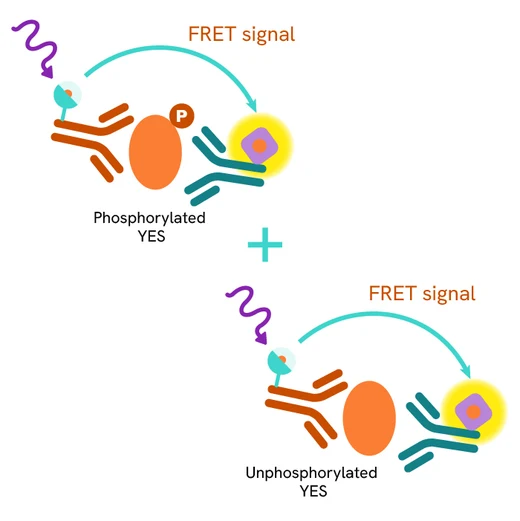
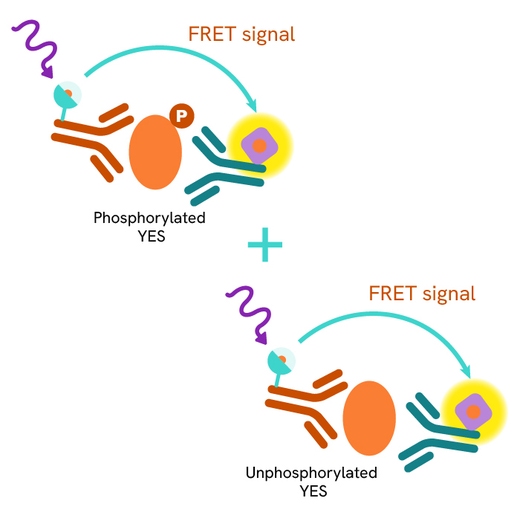
The Total YES assay quantifies the expression level of YES in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total YES assay uses two labeled antibodies, one coupled to a donor fluorophore and the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In the presence of YES in a cell extract, these conjugates bring the donor fluorophore into close proximity with the acceptor, generating a FRET signal. The signal intensity is directly proportional to the concentration of the protein present in the sample and provides a means of assessing the protein's expression under a no-wash assay format

Total YES two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of HTRF Total YES detection reagents. This protocol allows for the cells' viability and confluence to be monitored.
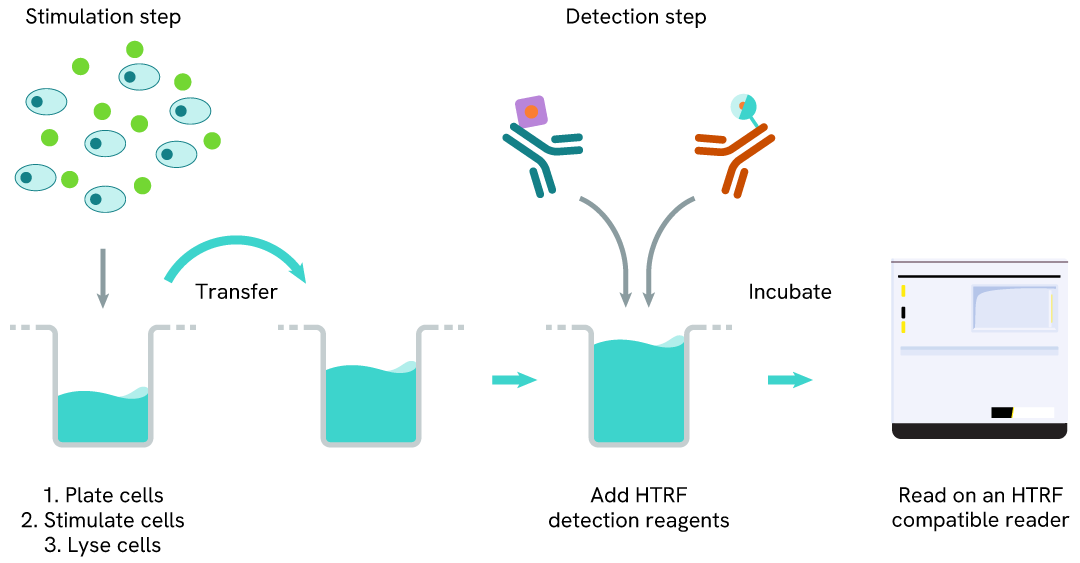
Total YES one-plate assay protocol
Detection of Total YES with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol allows miniaturization while maintaining robust HTRF quality.

Assay validation
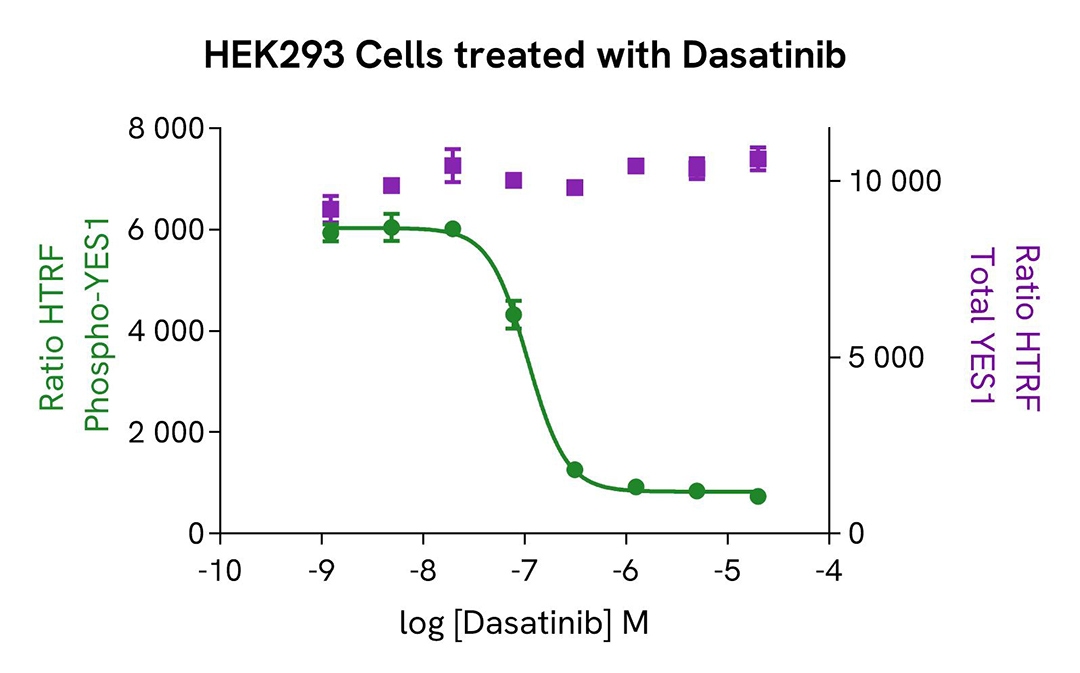
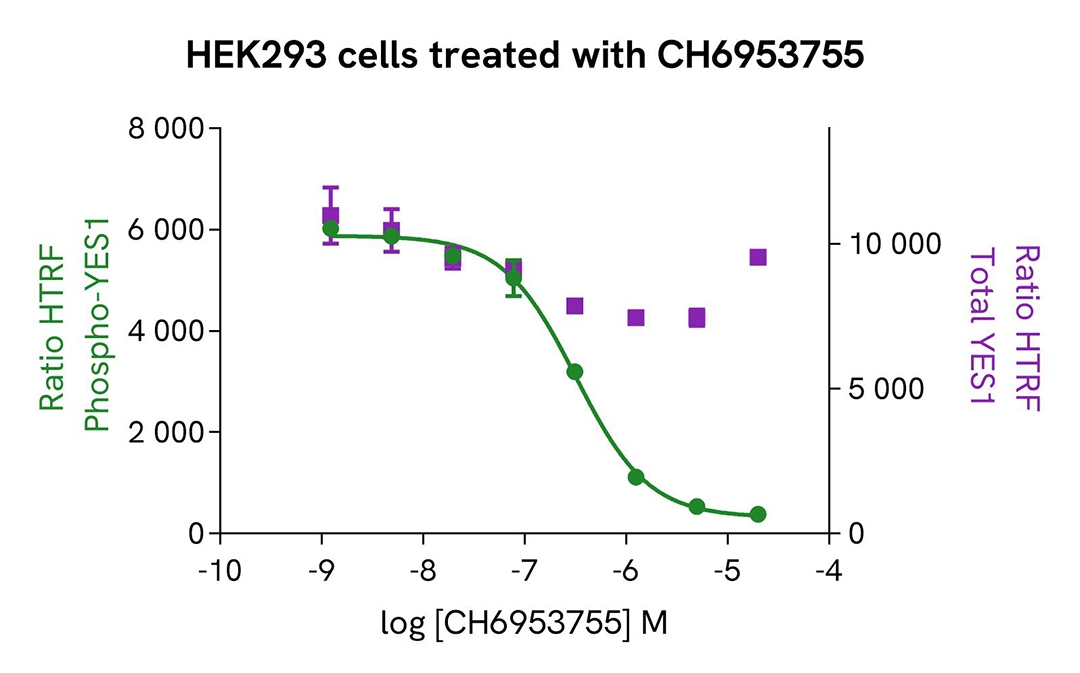
Inhibition of phospho-YES (Ty426) in HEK293 cells
HEK293 cells were seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. The cells were treated for 1.5 h with increasing concentrations of CH6953755,
described to be an SF inhibitor, and 30 µM of Pervanadate were added 30 minutes before the end of the treatment. The cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking.
For the detection step, 16 µL of cell lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Phospho-YES (Tyr426) or Total-YES detection reagents were added. The HTRF signal was recorded after 4h.
As expected, the Src kinase inhibitor CH6953755 induced a dose-dependent decrease in YES phosphorylation, without significantly impacting the expression level of the protein, while no toxicity was detected (ATPlite Luminescence Assay System, #6016943).
HeLa cells were seeded in a 96-well culture-treated plate (100,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. The cells were treated for 4 and 24 hours with increasing concentrations of DAS-5-oCRBN, a Src family PROTAC. For the shortest treatment, a co-treatment with epoxomicin at 1µM was done.
The cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. For the detection step, cell lysates were transferred into a 384-well low volume white microplate. 4 µL of the HTRF Total-YES or HTRF Total-c-Src detection reagents were added. The HTRF signal was recorded after 4h.
As expected, the Src Family PROTAC, DAS-5-oCRBN, induced a dose-dependent decrease in YES and c-Src expression. The kinetic of degradation was similar for both proteins. The presence of epoxomicin stopped the degradation, which demonstrated the proteasome involvement in the DAS-5-oCRBN induced process. Its effect was partial and increased over time. A slight decrease in cell viability was observed with the 24 hour treatment and on the highest PROTAC concentrations. After 4 hours of treatment, 25% of total YES and 53% of Src were degraded.
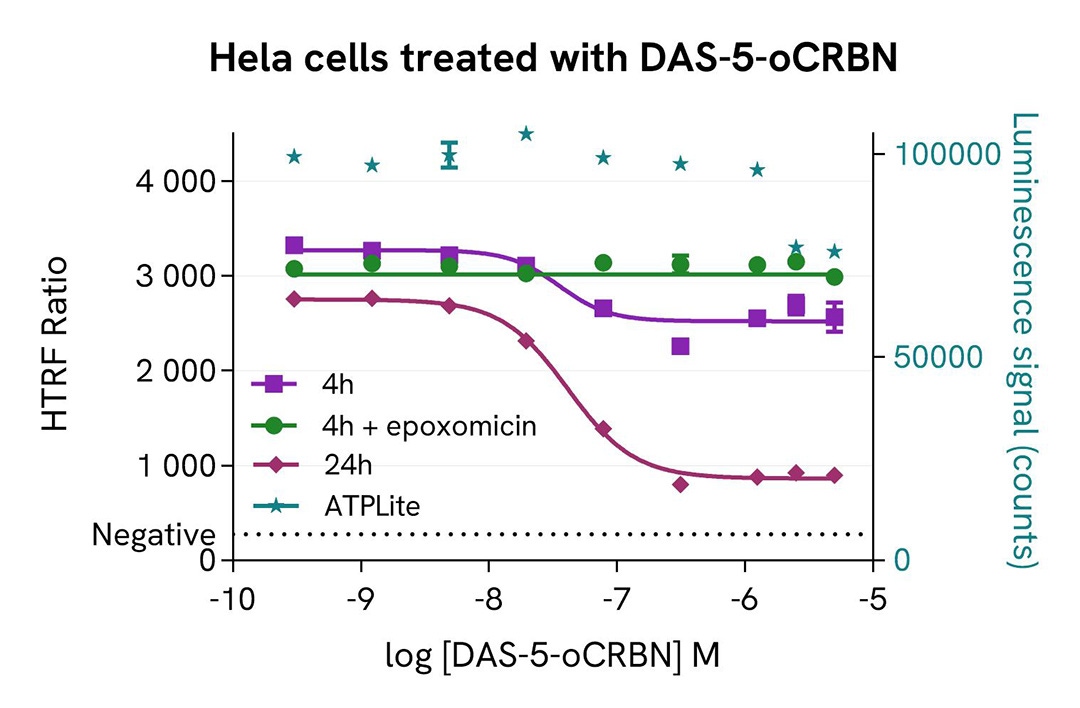
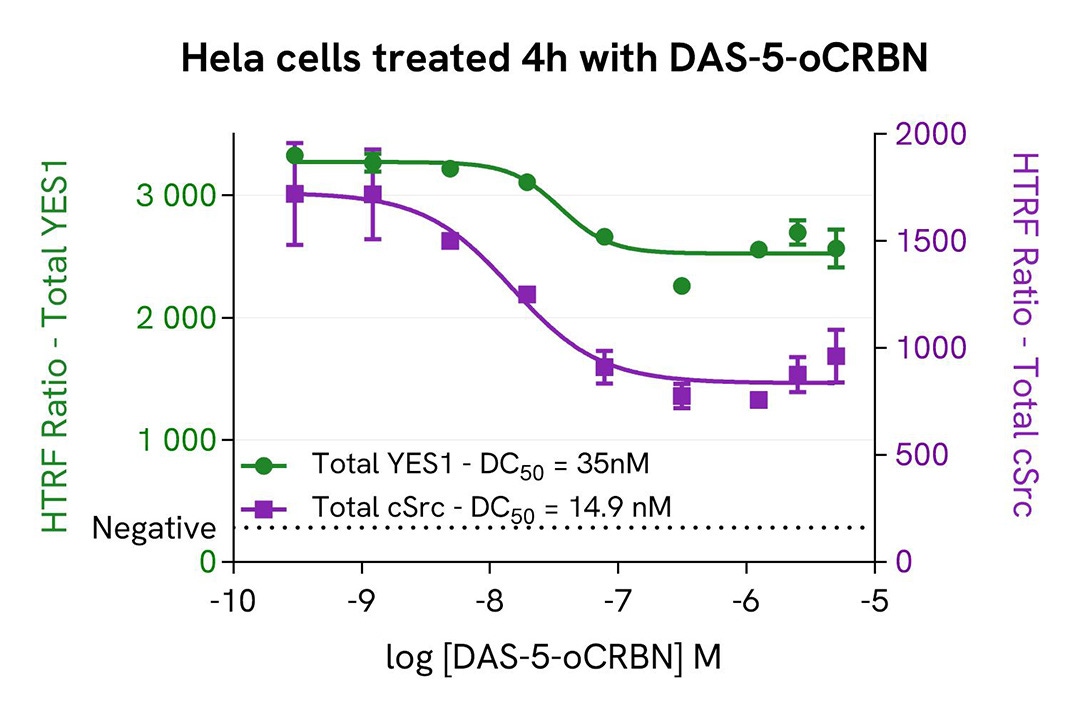
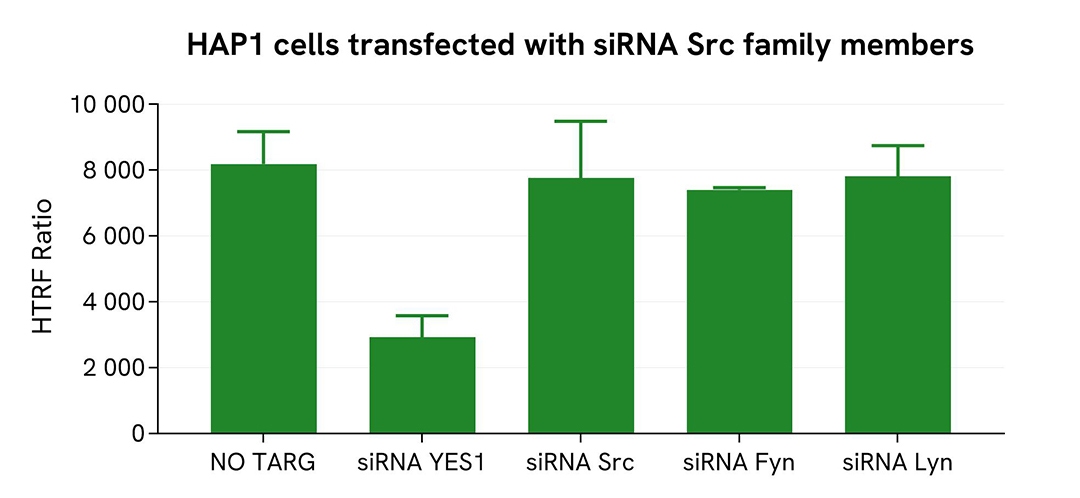
Validation of the selectivity of Total YES assay using siRNA Src Family
HAP1 cells were plated in a 96-well plate (100,000 cells/well) and cultured for 24h. The cells were then transfected with different siRNA, YES, Src, Fyn or Lyn, as well as with a negative control. Following a 24h incubation, the cells were lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking.
For the detection step, 16 µL of lysates were transferred into a 384-well low volume white microplate and 4 µL of the HTRF Total YES detection antibodies were added. The HTRF signal was recorded after 4 hours.
Cell transfection with siRNA YES led to a significant decrease (70%) in the detection of the protein compared to the negative control (non targeting control). On the contrary, the transfection of other Src family members (Src, Lyn, and Fyn) did not induce any signal modulation, demonstrating that the HTRF Total YES assay is selective for YES and does not cross-react with other family members.
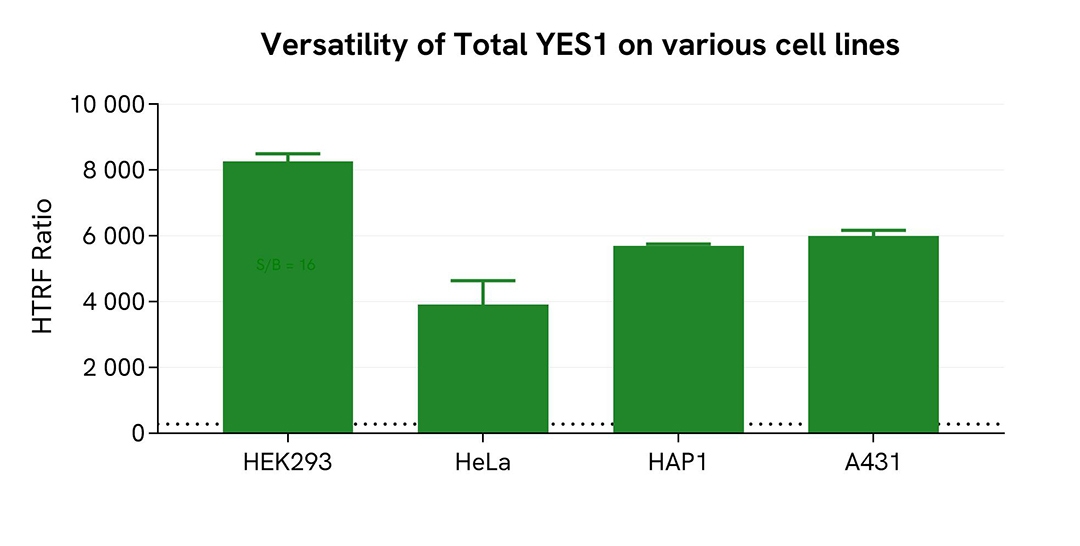
Assessment of Total YES level in various cell lines
HEK293, HeLa, HAP1, and A431 human cells were seeded at 100,000 cells/well in a 96-well microplate. After 24h of incubation, the cells were treated for 30 min with 30 µM of Pervanadate before being lysed for 30 minutes with supplemented lysis buffer #4, following the protocol for adherent or suspended cells, at RT under gentle shaking.
16 µL of lysate were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Total-YES detection reagents. The HTRF signal was recorded after an 4h incubation.
The HTRF Total-YES assay detected the protein in human various cellular models with different expression levels.
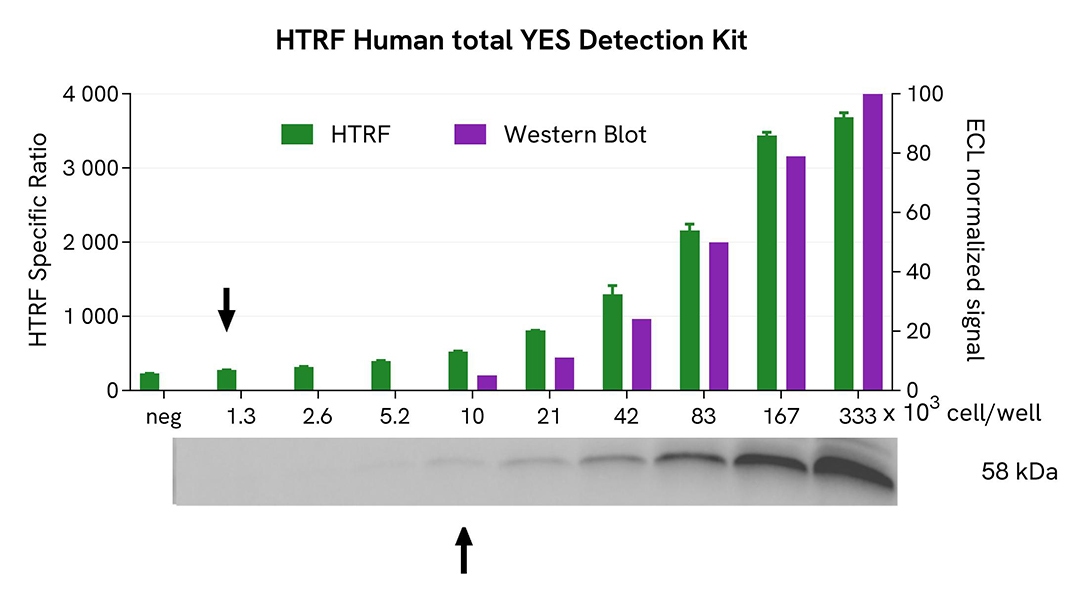
HTRF Total YES assay compared to Western Blot
Hela cells were grown in a T175 flask in complete culture medium at 37°C, 5% CO2 until 80% confluence. Cells were then lysed with 3 mL of supplemented lysis buffer #4 (1X) for 30 minutes at RT under gentle shaking.
Serial dilutions of the cell lysate were performed using supplemented lysis buffer, and 13 µL of each dilution were transferred for both detections. For HTRF detection, 13µL were transferred into a white 384-well microplate before the addition of 3 µL of supplemented lysis buffer #4 (1X). 4 µL of HTRF Total-YES detection reagents were then added. Equal amounts of lysates were used for a side-by-side comparison between HTRF and Western Blot.
Using the HTRF Total-YES assay, 1, 300 cells/well were enough to detect a significant signal, while 10,000 cells/well were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, in these conditions, the HTRF Total-YES assay was 8 times more sensitive than the Western Blot technique.
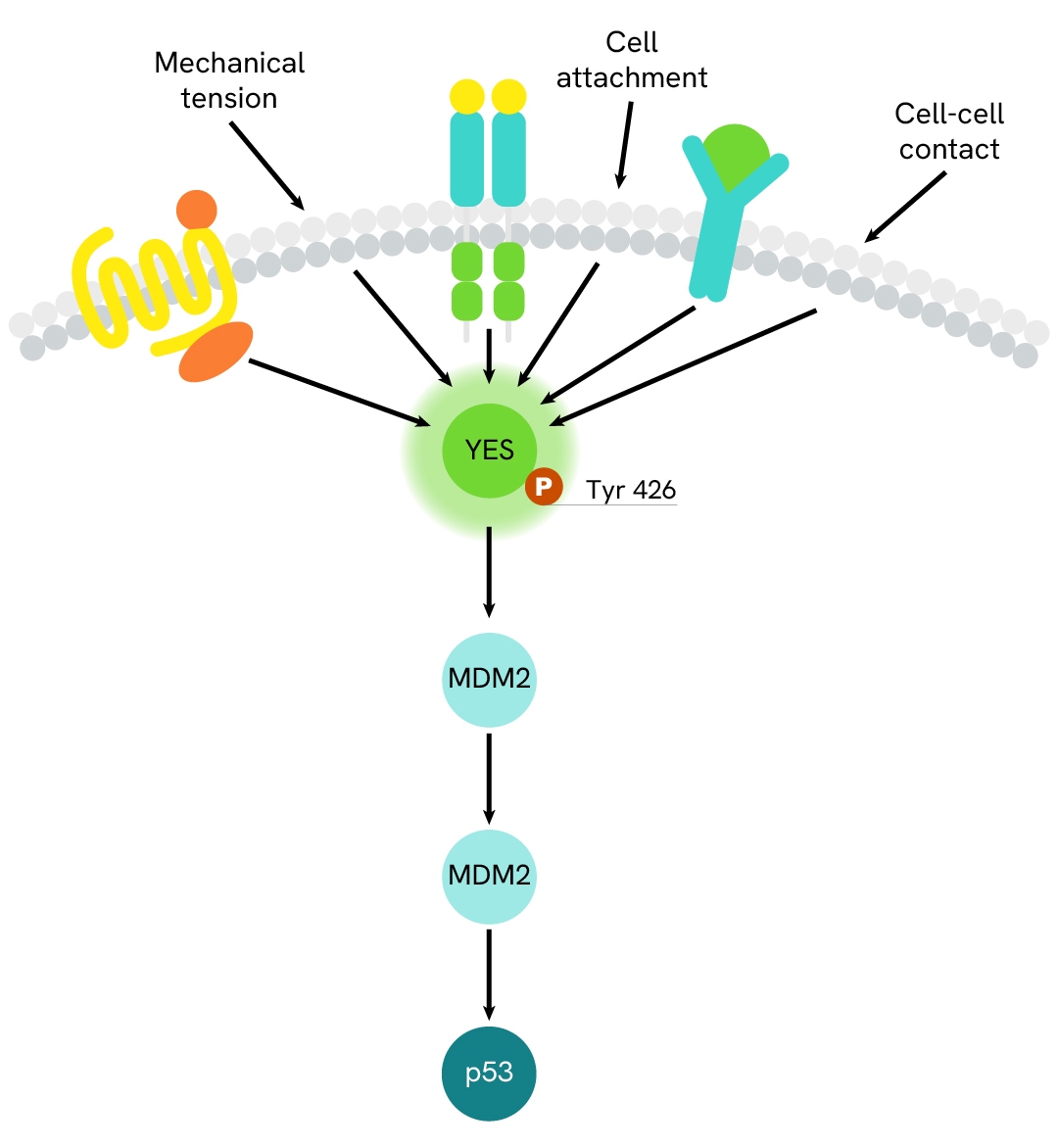
Simplified pathway
YES signaling pathway
YES, which belongs to the Src family of kinases, is a proto-oncogene and a non-receptor tyrosine kinase located at the plasma membrane. Once activated, YES phosphorylates various downstream effectors including LATS1/2, YAP, FAK, Caveolin-1, and p130CAS, as well as Crk. The kinase contributes to a wide range of cellular processes, including cell proliferation, survival, cytoskeleton rearrangements, migration, and adhesion. Dysregulation of YES can contribute to pathological conditions, especially in the context of cancer.
Loading...


Recently Viewed

How can we help you?
We are here to answer your questions.